Target Product Profile
Our company has adopted a business ethical decision to develop and market only products that are safe, high quality and have a significant impact on human wellness and health.
All our products are designed based on published and validated scientific research data with composition and, most importantly, sufficient dosing to meet the daily requirement delivering the desired benefits. This product profile criteria are very different to the majority of the supplements in the market which only include a fraction of the optimal dosage to allow them label their products with the said ingredients without delivering the expected benefits.






Product Portfolio
Our company continuously identifies important supplement products and trialing optimal formulations to deliver new products which would significantly impact on the health and wellness in human and their “companions”. Therefore, in addition to the current NMN line of products, which was recognized to be a superior supplement range, there are over 10 products under design and user trials. Some of these new pre-launch products have already generated very positive results amongst the initial users.
Capsule vs Tablet formulation
As has been known for many decades, how a supplement/medicinal product is formulated will determine the “oral bioavailability” of the product after consumption. Oral bioavailability refers to the amount and speed of each of the components in the product would be absorbed and enter the body after ingestion.
For product like NMN, it was observed that NMN was rapidly degraded in acid environment such as in the stomach (pH≈1-3). Most NMN supplements in the market is sold and packaged in half:half assembled normal or acid-resistant capsules. However, except fully sealed capsules, these half:half capsules are softened with a sticky surface inside the stomach which may stick to the stomach lining and pulled open by the stomach churning motion. This is often characterized by the discomfort and taste experienced by users during the 30 minutes whilst the capsules remained in the stomach before ejected into the intestine for further digestion. Once opened, the content inside the capsule was released and subject to degradation by stomach acid.
Another example is the supplement resveratrol, which is a hydrophobic (fat-like) substance and does not dissolve in water-based environment easily (solubility in water is 30mg/litre. When resveratrol is dissolved in water, the powder will stick together to form lumpy aggregates which would be hard to disperse and absorbed in the intestine.
As part of the product development, our decision is to choose the more suitable delivery method using the pressed tablet formulation. The tablet offers two advantages for the NMN product range: firstly, the tablet is coated and so compressed that the tablet does not dissolve in the stomach until it reaches the intestine released gradually. Secondly, the NMN and the synergistic components (e.g. resveratrol or epimedium extract) are pre-mixed and dispersed. When the tablet is dissolving, the resveratrol in the tablet is dissolved gradually without the opportunity to form large aggregates, hence assist in its dissolution and absorption through the intestine into the bloodstream.

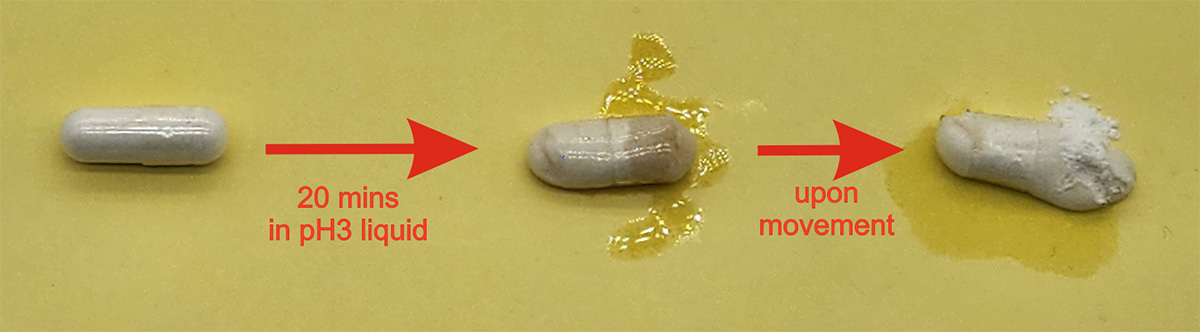
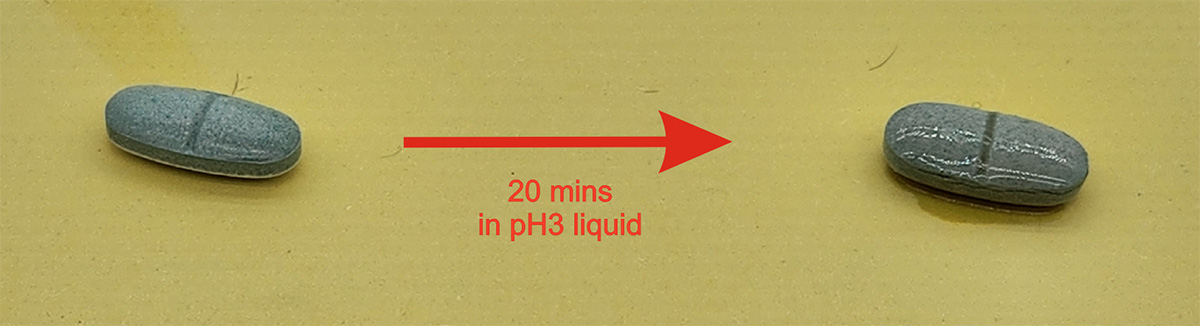
Efficacy
We test each product’s efficacy according to the Target Product Profile (TPP) before the product is commercialized and launched. The objective is to ensure the product is used by customers and experience the benefits to their health according to the expected benefits from the ingredient dosage and the product’s description.
Product Manufacturing
All our products use high quality ingredients and are manufactured in accredited production facilities owned by reputable multi-national enterprises meeting specific international standards, such as Good Manufacturing Practice. These facilities are routinely validated by third party inspection organisations.


